Primary Structure:
Every protein has a particular sequence of amino acids. The primary structure is important because many genetic diseases result in proteins with abnormal amino acid sequence.
- Peptide Bond:
Amino acids are joined covalently by peptide bonds. Link between alpha carboxyl group of one amino acid and alpha amino group of another. Peptide bonds are not broken by conditions that denature proteins. To hydrolyze these bonds non-enzymatically it is required that exposure to a strong acid or base is given at elevated temperatures.
- Characteristics of peptide bonds:
Peptide bond has a partial double bond character, shorter than a single bond and rigid and planar. No free rotation around the bond between the carbonyl carbon and the nitrogen of the peptide bond. Rotation takes place in the bonds between alpha-carbon and alpha-amino or alpha-carbon and alpha-carboxyl groups. Peptide bond is generally trans and not cis because of steric interferences of the R-groups.
- Polarity of the peptide bond:
C=O and NH groups of the peptide bond are uncharged. Charged groups are only the N-terminals of Alpha amino group, the C-terminal (alpha carbonyl) group and any ionized group present in the side chains of the constituent amino acid.
- Sequencing of the peptide from its N-terminal:
The step wise process of identifying the specific amino acid at each position in the peptide chain starting at N-terminal end is called sequencing.
Secondary Structure:
Amino acids are located in such a way that they lie close to each other in the linear sequence. It takes up three forms Alpha-helix, Beta-sheet and Beta bend.
Alpha-Helix:
 This is the most common form of secondary structure. It is a spiral structure having tightly packed, coiled polypeptide back bone core. Keratin structure is nearly entirely Alpha-helical. Keratin is rigid. Rigidity determined by the number of disulphide bonds between the constituent polypeptide chains. Myoglobin is also made of Alpha-helix but it is globular and flexible molecule.
This is the most common form of secondary structure. It is a spiral structure having tightly packed, coiled polypeptide back bone core. Keratin structure is nearly entirely Alpha-helical. Keratin is rigid. Rigidity determined by the number of disulphide bonds between the constituent polypeptide chains. Myoglobin is also made of Alpha-helix but it is globular and flexible molecule.Hydrogen Bonds;
The hydrogen bonding between the oxygen of carbonyl group and the hydrogen of amide stabilizes the Alpha-helix. The hydrogen bond forms between the carbonyl oxygen of one peptide bond to the NH-group of a peptide linkage 4 residues ahead in the polypeptide chain. Hydrogen bonds individually are weak but collectively form a stable Alpha-helix. Proline disrupts an Alpha-helix as its secondary amino group is not geometrically compatible with the right handed spiral of the alpha-helix. It inserts a kink into the chain. Charged amino acids can also disrupt the helix by forming ionic bonds or by repelling each other. Bulking and amino acid branching at Beta-carbon if present in large numbers can affect the formation of helix.
Beta-sheet:
 All peptide bond components are involved in hydrogen bonding. Surface of Beta sheets is pleated. Often called Beta pleated sheets. Unlike Alpha-helix, beta-sheets are composed of two or more peptide chains or segments of polypeptide chains. in beta sheet hydrogen bonds are perpendicular to the backbone, in Alpha-helix hydrogen bonds are parallel to the backbone. When hydrogen bonds are formed between the polypeptide backbones of seperate polypeptide chains, they are termed interchain bonds. When formed by single polypeptide chain folding back on itslef then the hydrogen bonds are intrachain bonds. Polypeptide chain may be arranged in parallel or anti-parallel manner. If hydrogen bond formed between polypeptide backbones of separate polypeptide chains then this is interchain bond. If a single polypeptide chain folds on itsself then the hydrogen bond here is intrachain bond. In globular proteins Beta-sheets have a right handed curl when viewed along the polypeptide backbone.
All peptide bond components are involved in hydrogen bonding. Surface of Beta sheets is pleated. Often called Beta pleated sheets. Unlike Alpha-helix, beta-sheets are composed of two or more peptide chains or segments of polypeptide chains. in beta sheet hydrogen bonds are perpendicular to the backbone, in Alpha-helix hydrogen bonds are parallel to the backbone. When hydrogen bonds are formed between the polypeptide backbones of seperate polypeptide chains, they are termed interchain bonds. When formed by single polypeptide chain folding back on itslef then the hydrogen bonds are intrachain bonds. Polypeptide chain may be arranged in parallel or anti-parallel manner. If hydrogen bond formed between polypeptide backbones of separate polypeptide chains then this is interchain bond. If a single polypeptide chain folds on itsself then the hydrogen bond here is intrachain bond. In globular proteins Beta-sheets have a right handed curl when viewed along the polypeptide backbone.Beta-bend:
 Beta-bend reverses the polpeptide chain direction and helps form a compact, globular shape. Beta-bend generally have four amino acids. One of these may be the Proline that causes kink in the polpeptide chain. Glycine is also found in Beta-bends. Hydrogen and ionic bonds stabilize Beta-bends.
Beta-bend reverses the polpeptide chain direction and helps form a compact, globular shape. Beta-bend generally have four amino acids. One of these may be the Proline that causes kink in the polpeptide chain. Glycine is also found in Beta-bends. Hydrogen and ionic bonds stabilize Beta-bends.
Non-competitive secondary structures (motifs) have a less regular structure. Super secondary Structured elements are produced by packing side chains from adjacent secondary structural elements close to each other.
Tertiary Structure:
Primary structure of a polypeptide chain determines its tertiary structure. Domains are fundamental, functional and 3D structural units of polypeptides. Length of polypeptide if greater than 200 aminoacids then it contains 2 or more domains. The core of domain is made up of motifs. 3D structure determined by the amino acid sequences. The side chain interaction in amino acids results in the formation of a compact structure. Disulphide bond formed from sulfhydryl group reactions. Disulfide bonds provide staility to the 3D shape and prevents the protein from becoming denatured in extracellular environment. Hydrophobic interactions that is the clustering of nonpolar side chains in the interior of the polypeptide chaian if in a polar environment and if in lipid environment then nonpolar side hcaians on the exterior. Hydrogen bonds can be formed with electron rich atoms by the aminoacids having oxyen or nitrogen bound hydrogen. Hydrogen bond formation between polar groups on protein surface and the aqueous solvent increases the protein solubility. Ionic interactions are the interactions between positively charged groups example amino groups and negatively charged groups example carboxylate groups. Denaturation is unfolding and disorganization of the protein's secondary and tertiary structures. Denaturing agents including heat, organic solvents, mechanical mixing, strong acids or bases, detergents and ions of heavy metals example lead and mercury. Denaturation may sometimes be reversible. protein begins to fold in stages during its synthesis. Also specialized groups of proteins called chaperones are required for proper folding of many proteins. Chaperones are also known as 'heat shock' proteins. Some chaperones keep the proteins unfolded till their synthesis is complete. They act as catalyst by increasing the rate of final stages in the folding process. Others protect the protiens so that their exposed region doesnot become tangled in unproductive interactions.
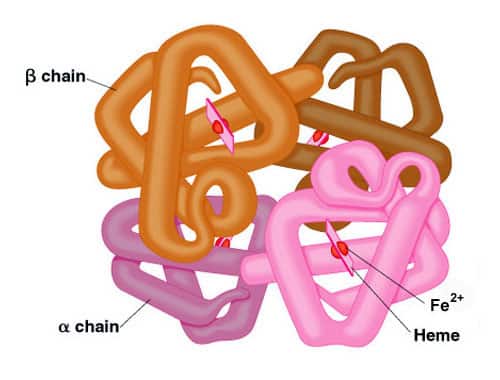 Quaternary Structure:
Quaternary Structure:
Monomeric proteins contain single polypeptide chains. Arrangement of polypeptide subunits is called Quaternary Structure of proteins. Interactions are non-covalent example hydrogen bonds, ionic bonds and hydrophobic interactions. Subunits may function independently or co-operatively. Isoforms are proteins performing the same functions but having different primary structures. If these isoforms function as enzymes then they are isoenzymes.
Diseases:
Amyloid Disease:
Insoluble, spontaneously aggregating proteins called Amyloid. Contributes to alzheimer's disease. In alzheimer's disease amyloid beta containing 40-42 amino acids accumulates. This peptide in a beta-pleated sheet configuration is neurotoxic. Accumulation of neurofibrillary tangles inside neurons also contributes to Alzheimer's disease. These tangled fibers form due to abnormal tau proteins. these in healthy versions help assembling microtubular structures. But if defective blocks the action of its normal version.
Prion protein:
Causative agent of transmissible spongiform encephalopathies. Infective PrP is resistant to proteolytic degradation. The only difference in normal and infective PrP is that a few Alpha helices are replaced by Beta sheets.


No comments :
Post a Comment