- only 20 Amino Acids are commonly found as constituents of mammalian proteins.
- Every amino acid has a carboxyl group, a primary amino group and a distinctive side chain.
- The nature of the side chain ultimately dictates the role an amino acid plays in a protein.
- Amino acids are classifieds according to the properties of their side chains.

Amino Acids with non polar side chains:
- No lose or gain of protons by the side chain.
- These are said to be oily or lipid like side chains thus they are hydrophobic.
- In the aqueous solutions the non polar side chains of Amino acids in proteins tend to cluster in the interior of the protein.
- This is Hydrophobic effect.
- The clustering in the interior helps give the protein its 3D structure.
- In the hydrophobic environment these R-groups are found on the outside of the proteins.
- Problems can occur if a polar R group is replaced by a non-polar R group like in sickle cell anemia.
Proline:
- A 5 member ring structure is formed by the interaction of the side chain and alpha amino N.
- Has secondary amino groups.
- It is called imino acids.
- Geometry of proline contributes to the formation of collagen.
Amino Acids with uncharged polar side chains:
- Zero net charge at neutral pH.
- The hydroxyl group of serine, threonin and tyrosine. The carbonyl and amino groups of asparagine and glutamine form hydrogen bonds.
Disulphide bonds:
- When the SH groups of two cysteines becomes oxidized to form a dimer, cystine is formed which contains disulphide bonds.
- The sulfhydrl SH group is often an important part of an active site.
Side chains as site of attachment for other compounds:
- The polar hydroxyl groups of serine, threonine and rarely tyrosine serve as attachment sites for sturctures such as phosphate group, oligosaccharide chains.
Amino Acids with acid side chains:
- Proton donors.
- Fully ionized at physiologic pH.
- Contains negatively charged carboxylate group (-COO)
Amino Acids with basic side chains:
- Side chains accept protons.
- At physiologic pH fully ionized and positively charged side chains.
- Histidine is either neutral or positively charged depending on the ionic environment the polpeptide chains or proteins provide it.
Optical Properties:
- alpha carbon is a chiral or optically active carbon atom. Glycine with two hydrogens is an exception.
- Dextrorotatory (D) and levorotatory (L) are mirror images of each other.

- Amino acids forming proteins are of L-configuration.
- D- amino acids in antibiotics, plants and bacterial cell walls.
Acidic and basic properties:
- Acidic are proton donors.
- Bases are proton acceptors.
- Free amino acids and peptides linked amino acids both can act as buffers because in addition to acidic and basic alpha carbonyl and alpha amino groups they also contain ionizable group in its side chain.
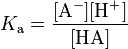
- Larger the dissociation constant, stronger the acid
Buffers:
- Resists changes in pH when an acid or base is added.
- Buffer= weak acid + conjugate base.
- Maximum buffering capacity occurs at pH equal to pKa.
- HA = A- then the pH equal to pKa.
- At pH value of less than pKa, protonated acid or weak acid is predominant, at pH value of more than pKa the deprotonated base ar conjugated base form is predominant.
- At low pH both the carboxly group and amino groups are protonated. As the pH is raised the COOH group dissociates to form carboxylate group -COO. this in turn forms a dipolar structure that is zwitterion.
- zwitterion has a net charge of zero.
- Dissociation constant smaller, pKa larger.
- Isoelectric point is the pH at which an amino acid is electrically neutral. Sum of positive charges = Sum of negative charges.
- Amino acids can act either as an acid or as a base thus is defined as amphoteric and are called ampholytes (amphoteric elecrolytes)
- Drug passes membranes more readily if uncharged.
- Effective concentration of drug determined by the relative amount of charged and uncharged forms.
Amino acids useful as drugs:
- Non standard amino acids used as drugs.
- A metabolite of penicillin, D-penicillamine is employed inthe chelation therapy of wilson's disease.
- N-acetylcysteine used in cystic fibrosis and chronic renal insufficiency.
- Gabapentin (gamma aminobutyrate linked to cyclohexane) is used as an anticonvulsant.
- Every life process depends on proteins example: enzymes and polypeptide hormones.
- proteins are polymers of amino acids.
No comments :
Post a Comment